Understanding Modafinil Non-Response: A Comprehensive Examination of Potential Causes
1. Introduction: The Enigma of Modafinil Non-Response
The experience of an individual taking a standard 200mg daily dose of modafinil and perceiving no discernible effect is a pertinent issue in the study of psychopharmacology. While modafinil is recognized for its wake-promoting and potential cognitive-enhancing properties, a variable response among users is a well-documented phenomenon. The observation that some individuals do not respond to modafinil is not an isolated occurrence but a recognized aspect of its clinical profile, suggesting a multifactorial basis for such variability.1 A lack of therapeutic effect, or "non-response," can stem from a complex interplay of factors unique to each individual. These factors encompass how the body absorbs, distributes, metabolizes, and excretes the drug (pharmacokinetics), how the drug interacts with its biological targets to produce effects (pharmacodynamics), inherent genetic predispositions that alter these processes, external influences such as concomitant medications or substances, the presence of underlying medical or psychological conditions, and even subjective elements related to expectation and perception of the drug's effects. This report will undertake a detailed exploration of these multifaceted causes, drawing upon scientific literature to elucidate why modafinil may not exert its expected effects in certain individuals. The mixed results observed in various clinical studies and investigations into cognitive enhancement further underscore that a lack of efficacy is a recognized challenge, necessitating a systematic approach to understand its origins.3
2. Modafinil: Mechanisms and Expected Outcomes
Understanding why modafinil might not work for some individuals begins with an appreciation of its complex pharmacology and the range of its intended effects.
Elucidating Modafinil's Complex and Not Fully Understood Mechanism of Action
The precise mechanisms through which modafinil exerts its effects are still considered "elusive" or "not clearly established" by the scientific community, contributing to its classification as an "atypical" central nervous system (CNS) stimulant.2 This atypical nature suggests its actions are more nuanced and potentially subject to greater individual neurochemical differences compared to classical stimulants with more definitively characterized targets. This inherent complexity may contribute to a broader spectrum of responses, including instances of non-response.
Key Insight: Modafinil's atypical and multifaceted mechanism is a primary reason for the variability in user response, including non-response if an individual's neurochemistry isn't suitably aligned.
Modafinil's activity involves multiple neurotransmitter systems:
- Dopamine (DA): A primary and clinically important property of modafinil is its function as a weak but selective dopamine transporter (DAT) inhibitor. This action reduces the reuptake of dopamine into presynaptic neurons, thereby increasing extracellular dopamine concentrations in certain brain regions.6 The interaction of modafinil with DAT is conformationally distinct from that of classical stimulants like cocaine, which may account for its lower potential for abuse and different subjective effects.8 The pharmacodynamic response related to dopamine elevation is notably protracted, meaning it is sustained over a longer period compared to other stimulants.9
- Norepinephrine (NE): Modafinil appears to have little to no direct affinity for norepinephrine transporters.8 However, it may indirectly elevate norepinephrine levels, possibly as a consequence of increased dopamine activity or through other mechanisms.5 Some earlier theories also proposed a modulation of central alpha-1 adrenergic receptors.1
- Serotonin (5-HT): Similar to norepinephrine, modafinil shows little direct interaction with serotonin transporters.8 Indirect upregulation of serotonin systems has been suggested, and some of modafinil's effects on other neurotransmitters, like GABA, might be mediated via serotonergic pathways.5
- Histamine: There is evidence to suggest that modafinil may increase histamine release or enhance histaminergic signaling in the brain, which is a known wakefulness-promoting pathway.1
- Orexin/Hypocretin: Modafinil may augment signaling within the hypothalamic orexin (also known as hypocretin) system. Orexin neurons play a crucial role in promoting wakefulness and regulating arousal states, and their activation by modafinil is thought to contribute significantly to its wake-promoting effects.5
- Gamma-aminobutyric acid (GABA): Modafinil may reduce the release of GABA, an inhibitory neurotransmitter, or otherwise modulate GABAergic tone in the brain.1 This reduction in inhibitory signaling could contribute to increased neuronal excitability and wakefulness. These effects on GABA might be indirect, possibly mediated through its actions on serotonin or norepinephrine systems.7
- Glutamate: An increase in glutamate release or activation of glutamatergic circuits has also been associated with modafinil administration.1 Glutamate is the primary excitatory neurotransmitter in the CNS, and enhanced glutamatergic activity can lead to increased arousal and cognitive function.
The multifaceted interactions of modafinil with these diverse neurotransmitter systems underscore why its overall effect can vary significantly among individuals. If a person's baseline neurochemical environment is not suitably aligned with modafinil's subtle and widespread modulatory actions, they may not perceive a significant effect, particularly at a standard dosage like 200mg.
Defining the Expected Therapeutic and Off-Label Effects
Modafinil is primarily recognized and licensed for its wakefulness-promoting properties. It is used in the treatment of narcolepsy, obstructive sleep apnea (OSA) to address residual excessive daytime sleepiness (EDS) despite primary treatment (e.g., CPAP), and shift work sleep disorder (SWSD).6
Beyond these approved indications, modafinil is widely used "off-label" by healthy individuals seeking cognitive enhancement. Users often report improvements in attention, executive functions (such as planning and decision-making), learning, and memory.3 However, the scientific evidence for cognitive enhancement in healthy, non-sleep-deprived individuals is inconsistent.3 Some studies report benefits, while others find limited or no effects on various cognitive domains, or even impairments in specific areas like creative thinking or response speed.
Important Distinction: There's often a noticeable difference between modafinil's effects on wakefulness versus its impact on cognitive enhancement. A lack of expected cognitive improvement, even with increased alertness, can lead to perceived ineffectiveness.
A critical observation from research is the potential dissociation between modafinil's effects on wakefulness and its impact on cognitive enhancement.17 Some individuals may experience increased alertness or reduced fatigue without a corresponding significant improvement in cognitive performance, or vice-versa. This suggests that the neurobiological pathways mediating these two types of effects might be, to some extent, distinct and subject to different influencing factors, such as genetic variations (e.g., in the COMT gene). If an individual's primary expectation is cognitive improvement, a lack of this specific benefit—even if subtle wakefulness effects are present—could lead to the perception that the drug is "not working."
3. Pharmacokinetic Variables: The Body's Processing of Modafinil
Pharmacokinetics, which describes how the body absorbs, distributes, metabolizes, and excretes a drug, plays a fundamental role in determining its efficacy. Individual variations in these processes can lead to significant differences in drug exposure and, consequently, response.
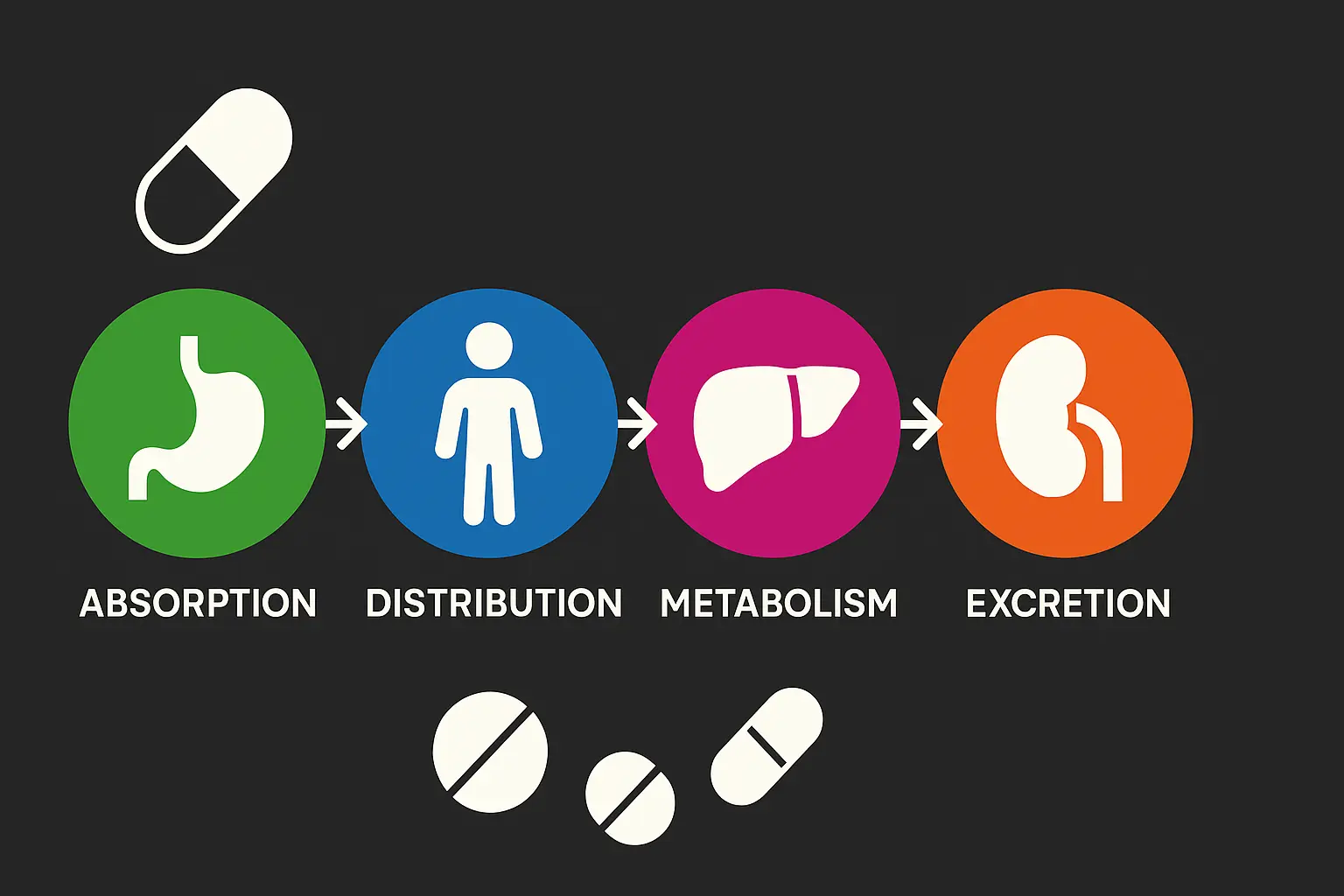
Absorption
Modafinil is generally readily absorbed following oral administration. Peak plasma concentrations (Cmax) are typically achieved within 2 to 4 hours after ingestion.7 The oral bioavailability of modafinil, which is the fraction of the administered dose that reaches systemic circulation, is estimated to be between 45% and 65%, based on urinary recovery of the drug and its metabolites.10 This range implies that a substantial portion of an ingested dose may not become systemically available to exert its effects. If an individual consistently has bioavailability at the lower end of this range (e.g., 45%), a 200mg oral dose would result in an effective systemic exposure equivalent to only 90mg. This reduced systemic availability, potentially in combination with other factors like rapid metabolism, could lead to plasma concentrations insufficient to produce a noticeable effect.
Critical Factor: Insufficient plasma concentrations, due to factors like low bioavailability or rapid metabolism, are a key reason for modafinil's ineffectiveness in some individuals.
Influence of Food Intake and Timing of Administration
The presence of food in the stomach does not appear to affect the overall bioavailability of modafinil. However, it can delay the time to reach peak plasma concentration (Tmax) by approximately one hour.8 For armodafinil (the R-enantiomer of modafinil), this delay in Tmax when taken with food can be more pronounced, around 2 to 4 hours.31 If modafinil is consistently taken with a meal, and an individual expects a rapid onset of action, this delay in achieving peak levels might lead to the perception that the drug is not working, especially if its effects are subtle for that person. Consistent timing of administration is generally recommended. For conditions like narcolepsy or OSAHS, a typical dose of 200mg is taken once daily in the morning. For SWSD, the dose is usually taken approximately one hour before the start of a work shift.22
Distribution
Once absorbed, modafinil distributes into body tissues. Its apparent volume of distribution is approximately 0.8 to 0.9 L/kg.10 Modafinil is moderately bound to plasma proteins, at a rate of about 60%, primarily to albumin.8 This degree of protein binding influences the amount of free (unbound) drug available to interact with target sites in the brain.
Hepatic Metabolism (Role of CYP450 enzymes)
Modafinil is extensively metabolized, primarily in the liver, with approximately 90% of the drug being eliminated through metabolic transformation.7 Less than 10% of an administered dose is typically excreted unchanged in the urine.8
The metabolism of modafinil involves several pathways, with amide hydrolysis being a major route, leading to the formation of modafinil acid. Cytochrome P450 (CYP) enzyme-mediated oxidative pathways also contribute, though to a lesser extent, to its overall metabolism.28 Modafinil itself interacts with several CYP enzymes: it is known to induce (increase the activity of) CYP1A2, CYP2B6, and CYP3A4/5.8 It also reversibly inhibits CYP2C1928 and has been shown to suppress CYP2C9 activity in vitro.28 Importantly, modafinil is also a substrate for metabolism by CYP3A4 and, to some extent, CYP2C19.33 Individual variability in the activity of these enzymes, often due to genetic factors (discussed later), can significantly impact how quickly modafinil is cleared from the body.
Renal Excretion
The primary metabolites of modafinil, modafinil acid and modafinil sulfone, are pharmacologically inactive with respect to wakefulness-promoting effects and are excreted mainly in the urine.7
Elimination Half-life and Onset of Action
The elimination half-life of modafinil is reported to be between 10 to 12 hours8 or 12 to 15 hours.7 This relatively long half-life is largely determined by the pharmacokinetics of the R-enantiomer (armodafinil), which is cleared more slowly than the S-enantiomer.10 Due to this half-life, steady-state plasma concentrations of modafinil are typically reached within 2 to 4 days of consistent daily dosing.28
Impact of Renal/Hepatic Impairment
In individuals with compromised kidney (renal) or liver (hepatic) function, the elimination of modafinil can be slowed down. Similarly, elderly individuals may experience reduced elimination rates due to age-related physiological changes, although typically to a lesser extent than those with significant organ impairment.7 Severe liver disease may necessitate a dose adjustment to prevent drug accumulation and potential toxicity.22
Potential Sex-Specific Differences in Pharmacokinetics
Emerging evidence suggests that there may be sex-specific differences in modafinil pharmacokinetics. Studies indicate that modafinil may have higher bioavailability in women compared to men. Following a single 200mg oral dose, the mean peak plasma concentration (Cmax) has been observed to be higher in women, and this difference can persist even after adjusting for body weight. Furthermore, the clearance of modafinil may be up to 30% higher in men than in women, resulting in significantly higher plasma concentrations in women after a single dose.8 If the individual in question is male, they might clear modafinil more rapidly, potentially leading to lower overall drug exposure from a standard dose compared to a female, which could contribute to a perceived lack of effect.
Summary of Pharmacokinetic Variability: Differences in absorption (bioavailability), metabolism (e.g., CYP enzyme activity, sex-specific clearance), and excretion (e.g., renal/hepatic impairment) are all key pharmacokinetic reasons for modafinil ineffectiveness in some individuals.
These pharmacokinetic characteristics highlight multiple points at which individual variability can lead to insufficient drug exposure. For example, an individual with lower-than-average bioavailability combined with a genetically determined rapid metabolism phenotype could clear a 200mg dose too quickly for therapeutic concentrations to be achieved or sustained, resulting in a lack of perceived effect.
4. The Genetic Blueprint: How Individual Biology Shapes Modafinil's Effects
Genetic variations play a crucial role in determining how individuals respond to medications, and modafinil is no exception. Polymorphisms (common variations) in genes encoding enzymes involved in neurotransmitter metabolism or drug metabolism can significantly alter modafinil's efficacy and perceived effects.

Catechol-O-Methyltransferase (COMT) Gene Variations
The COMT enzyme is responsible for the metabolic degradation of catecholamine neurotransmitters, including dopamine, epinephrine, and norepinephrine, particularly within the prefrontal cortex.17 A well-studied and common functional polymorphism in the COMT gene is Val158Met (rs4680). This single nucleotide polymorphism (SNP) results in a substitution of valine (Val) for methionine (Met) at codon 158 of the enzyme. The Val allele is associated with higher COMT enzyme activity (leading to faster dopamine breakdown), while the Met allele is associated with lower enzyme activity (slower dopamine breakdown).34
This genetic variation has a profound impact on modafinil response, particularly concerning its cognitive effects. Research has demonstrated that individuals with the COMT Val/Val genotype (higher enzyme activity, lower baseline prefrontal dopamine) tend to exhibit a significantly better response to modafinil in terms of improvements in sustained vigilant attention and executive functioning, especially under conditions of sleep deprivation. In contrast, individuals with the Met/Met genotype (lower enzyme activity, higher baseline prefrontal dopamine) may find modafinil "hardly effective" for these specific cognitive benefits.17 Interestingly, the wakefulness-promoting effects of modafinil may be experienced by individuals across different COMT genotypes, but the differentiation in cognitive enhancement is key.17 If an individual, such as the acquaintance mentioned in the query, is taking modafinil primarily for cognitive enhancement and possesses the COMT Met/Met genotype, they might accurately perceive the drug as "not working" for their intended purpose, even if subtle anti-fatigue effects are present. This dissociation highlights that different neurochemical pathways or sensitivities might underlie modafinil's alerting versus cognitive-enhancing actions. Furthermore, variations in COMT genotype (sometimes denoted by A or G alleles, which correspond to Val or Met depending on the specific SNP and allele definition) have been linked to differences in modafinil's effects on subjective vigor and well-being.19 The COMT Val158Met polymorphism also independently influences sleep-wake cycle characteristics, with Val/Val and Met/Met homozygotes showing a greater tendency to prolong sleep on rest days compared to Val/Met heterozygotes.37
Genetic Impact (COMT): Possessing the COMT Met/Met genotype is a significant genetic reason why some individuals find modafinil "hardly effective" for cognitive enhancement.
Cytochrome P450 (CYP) Enzyme Polymorphisms
CYP enzymes are critical for the metabolism of a vast number of drugs, including modafinil. Genetic polymorphisms in CYP genes can lead to significant inter-individual differences in enzyme activity, categorizing individuals as poor metabolizers (PMs), intermediate metabolizers (IMs), normal (extensive) metabolizers (NMs or EMs), or ultrarapid metabolizers (UMs).
CYP2C19:
This enzyme is involved in the metabolism of approximately 5-10% of all prescription drugs.38 Modafinil is known to inhibit CYP2C19 activity28 and is also a substrate for this enzyme, meaning CYP2C19 participates in its breakdown.33 Common genetic variants of CYP2C19, such as *2 and *3 alleles, lead to a loss of enzyme function (characteristic of PMs), while the *17 allele is associated with increased enzyme activity (characteristic of UMs).38 The prevalence of these variants differs across ethnic populations.38 If an individual is a CYP2C19 UM, they might metabolize modafinil (or a component of its metabolic pathway cleared by CYP2C19) more rapidly than normal. This accelerated clearance could result in lower plasma concentrations of modafinil and a shorter duration of action, potentially leading to reduced efficacy or a complete lack of perceived effect from a standard dose.
CYP3A4/5:
These are among the most important drug-metabolizing enzymes in humans, responsible for the clearance of a large proportion of clinically used medications.42 Modafinil induces CYP3A4 activity10 and is also metabolized by CYP3A4.8 Genetic polymorphisms affecting CYP3A4 and CYP3A5 activity exist and can influence the rate of drug metabolism.42 Similar to CYP2C19, individuals with genetically determined higher CYP3A4/5 activity (e.g., UMs or rapid metabolizers) could clear modafinil more quickly from their system. This would lead to lower systemic exposure to the drug, potentially rendering a standard 200mg dose sub-therapeutic and resulting in non-response.
It is also important to consider the concept of phenoconversion. An individual might possess a "normal metabolizer" genotype for relevant CYP enzymes, but concurrent use of other medications that induce these enzymes can effectively convert them into a "phenotypic" rapid metabolizer for modafinil. This acquired rapid metabolism, driven by drug interactions rather than baseline genetics, can also lead to sub-therapeutic modafinil levels and a lack of efficacy.44 This underscores that genetic testing alone may not predict response without considering the full context of an individual's medication regimen.
Polymorphisms in Dopamine System Genes
Given that modafinil's primary mechanism involves the dopamine system, genetic variations in components of this system can also influence its effects.
DAT1/SLC6A3 (Dopamine Transporter):
This gene encodes the dopamine transporter, modafinil's main molecular target. Polymorphisms in DAT1, such as a variable number tandem repeat (VNTR) in the 3'-untranslated region (associated with rs28363170), can affect dopamine regulation and have been linked to individual differences in baseline sleepiness and activity levels.37 For example, carriers of the 9-repeat (9R) allele of this VNTR have reported higher levels of daytime sleepiness.37 Variations in the DAT1 gene that alter the structure, expression level, or function of the dopamine transporter could directly impact modafinil's ability to bind to the transporter and inhibit dopamine reuptake. If an individual's DAT is less susceptible to inhibition by modafinil due to their genetic makeup, the drug's efficacy would likely be diminished. While some reviews note that evidence for the functional impact of certain DAT polymorphisms can be inconclusive36, its role as the primary target makes it a strong candidate for genetic influence on response.
DRD2 (Dopamine D2 Receptor):
This gene encodes the D2 dopamine receptor, a key postsynaptic receptor that mediates many of dopamine's effects. Polymorphisms in DRD2, such as the Taq1A polymorphism (rs1800497) and rs1076560, have been shown to influence D2 receptor binding characteristics and gene splicing.36 Dopamine D1 and D2 receptors are implicated in the development of behavioral sensitization to modafinil.45 Genetically determined differences in D2 receptor density, sensitivity, or signal transduction efficiency could alter the downstream consequences of modafinil-induced dopamine release, leading to a varied subjective or physiological response.
The following table summarizes key genetic polymorphisms and their potential impact on modafinil response:
| Gene (Polymorphism) | Typical Function of Gene Product | Variant Effect on Function | Potential Impact on Modafinil Response |
|---|---|---|---|
| COMT (Val158Met / rs4680) | Degradation of dopamine, norepinephrine, epinephrine | Val allele: higher enzyme activity (faster DA breakdown). Met allele: lower enzyme activity (slower DA breakdown). | Val/Val: Better cognitive response. Met/Met: Cognitive benefits "hardly effective," though wakefulness may be preserved. Affects vigor/well-being. |
| CYP2C19 (*2, *3, *17 variants) | Metabolism of various drugs, including modafinil (substrate role) | *2, *3: Loss-of-function (Poor Metabolizer - PM). *17: Gain-of-function (Ultrarapid Metabolizer - UM). | UM: Potentially faster modafinil clearance, leading to lower drug exposure and reduced efficacy. PM: Potentially higher exposure, risk of side effects (less likely non-response unless complex). |
| CYP3A4 (various functional variants) | Metabolism of many drugs, including modafinil (substrate role) | Variants can lead to increased or decreased enzyme activity. | Individuals with genetically higher CYP3A4 activity: Potentially faster modafinil clearance, lower exposure, and reduced efficacy. |
| DAT1/SLC6A3 (e.g., VNTR rs28363170) | Dopamine reuptake from synaptic cleft (modafinil's primary target) | Variants can affect DAT expression/function, influencing baseline dopamine regulation and sleepiness. | Altered DAT structure/expression could change modafinil's binding affinity or inhibitory efficiency, directly blunting its effect on dopamine levels. |
| DRD2 (e.g., Taq1A / rs1800497) | Postsynaptic dopamine receptor mediating DA effects | Variants can influence D2 receptor binding, density, or sensitivity. | Altered D2 receptor function could change the downstream cellular and behavioral responses to modafinil-induced increases in dopamine, leading to a different perceived effect or non-response. |
These genetic factors illustrate the intricate biological landscape upon which modafinil acts, providing plausible explanations for why a standard 200mg dose may be effective for one person but not another.
5. Pharmacodynamic Factors: Variability at the Target Site
Pharmacodynamics refers to what the drug does to the body, including its interactions with receptors and its biochemical and physiological effects. Individual differences at this level can also contribute significantly to variations in modafinil response.
Individual Differences in Receptor Sensitivity
The effects of modafinil are contingent upon the activation of dopamine receptors, among others.9 The sensitivity and density of these receptors, such as dopamine D1 and D2 receptors, are not static and can vary among individuals due to genetic factors, age, or prior exposure to substances that affect these systems. For instance, repeated administration of psychostimulants can lead to changes in dopamine receptor sensitivity, a phenomenon known as behavioral sensitization.45 If an individual possesses inherently lower sensitivity or a reduced number of relevant dopamine receptors in key brain circuits involved in wakefulness or cognition, a standard dose of modafinil might not elevate dopamine levels sufficiently, or the elevated dopamine might not trigger an adequate downstream signaling cascade to produce a perceptible effect. This concept of receptor plasticity means that an individual's dopamine system might be less responsive to begin with, requiring a stronger stimulus (e.g., higher modafinil dose or greater dopamine release) to achieve a noticeable outcome.
Baseline Neurotransmitter Levels and "Dopamine Tone"
The neurochemical state of an individual before taking modafinil can profoundly influence the drug's effects. This is often conceptualized by an "inverted U-shaped curve," particularly for dopamine's role in cognitive functions like those mediated by the prefrontal cortex.34
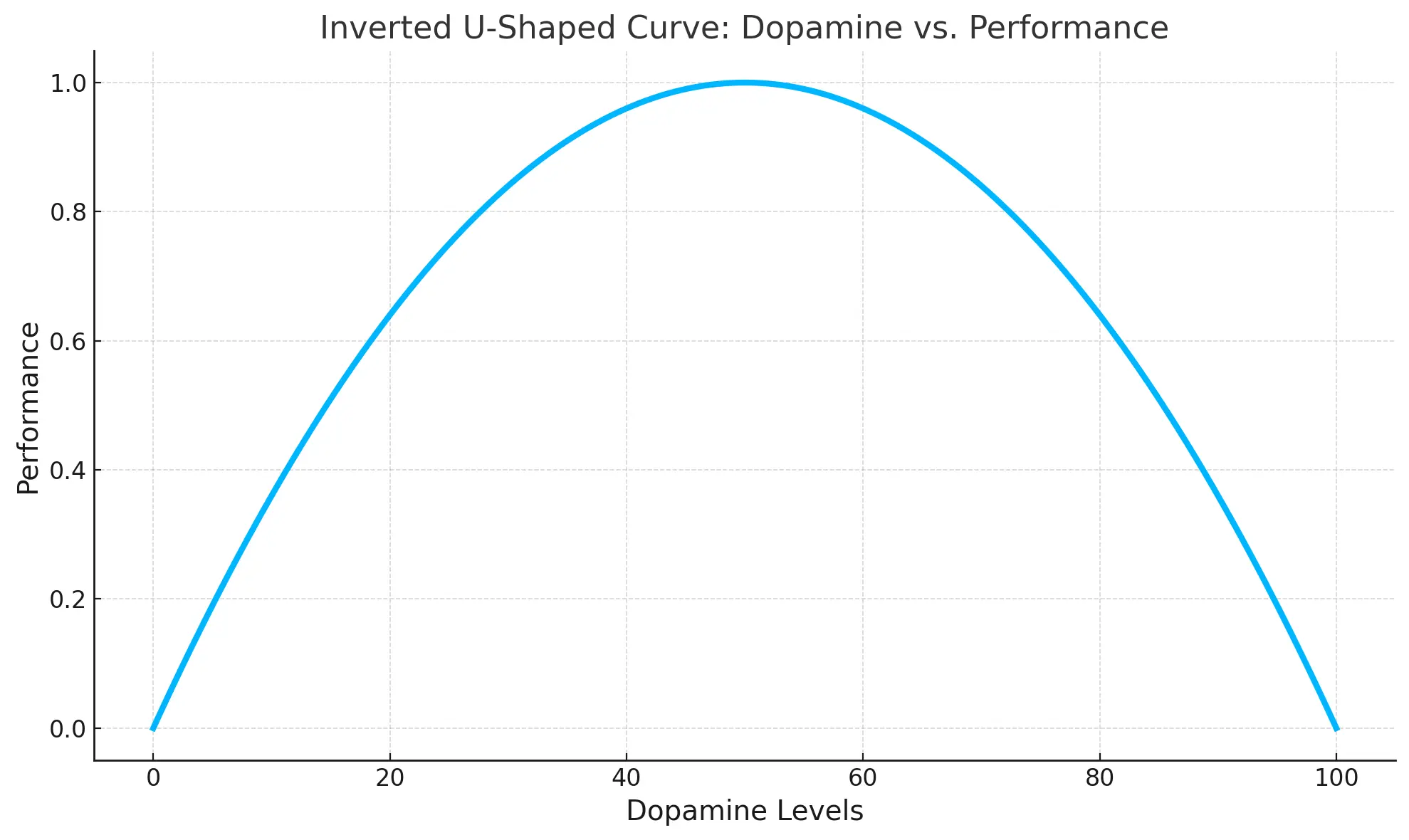
This model suggests that cognitive performance is optimal at a moderate level of dopamine activity; too little or too much dopamine can impair performance. Modafinil's efficacy, especially for cognitive enhancement, might therefore be more pronounced in individuals whose baseline dopamine levels are sub-optimal for a given task or state.3 If an individual already has high or optimal baseline dopamine levels in relevant brain circuits, modafinil (at a 200mg dose) might either offer no additional benefit or, theoretically, push dopamine activity beyond the optimal point, potentially leading to no improvement or even a slight decrement in function. This could be perceived as the drug "not working."
Similarly, modafinil is known to decrease cortical GABA release and can increase glutamate levels.15 An individual's baseline GABAergic tone or glutamatergic activity could interact with modafinil's effects, leading to different outcomes. For example, if baseline inhibitory GABA tone is already very low, the additional reduction by modafinil might not yield a significant change in arousal or cognition.
Pharmacodynamic Nuance: An individual's existing neurotransmitter balance (e.g., dopamine, GABA) and receptor sensitivity are critical pharmacodynamic reasons for why modafinil may not produce the desired effect.
Modafinil as an "Atypical" Stimulant: Implications for Perceived Effects
Modafinil's pharmacological profile is distinct from that of classical psychostimulants like amphetamines or methylphenidate. A key difference is the general lack of significant euphoric or intensely pleasurable effects with modafinil at therapeutic doses.7 The subjective experience of taking modafinil is often described as being more akin to that of caffeine rather than amphetamine.13 This "atypical" nature, combined with its protracted and relatively modest elevation of dopamine compared to classical stimulants9, results in a qualitatively different psychoactive experience. If an individual expects the pronounced "rush," mood elevation, or intense stimulation characteristic of traditional stimulants, the more subtle and nuanced effects of modafinil might be underwhelming and interpreted as the drug not working, even if it is providing some level of wakefulness promotion or cognitive support. This mismatch between expectation and modafinil's actual subjective profile is a significant potential contributor to perceived non-response, especially in off-label users seeking specific types of enhancement.
6. Drug Interactions: The Influence of Concomitant Substances
The efficacy of modafinil can be significantly altered by interactions with other substances, including prescription medications, over-the-counter drugs, herbal supplements, alcohol, and caffeine. These interactions can affect modafinil's pharmacokinetics (leading to altered drug levels) or pharmacodynamics (leading to altered effects).
Interactions with Prescription Medications (CYP450 Inducers/Inhibitors)
Modafinil itself is an inducer of several CYP450 enzymes (CYP3A4, CYP1A2, CYP2B6) and an inhibitor of others (CYP2C19).28 This means it can affect the metabolism of other drugs. More relevant to modafinil non-response, however, are drugs that affect modafinil's metabolism. Since modafinil is metabolized by CYP3A4 and to some extent by CYP2C1933, co-administration with drugs that are potent inducers of these enzymes can accelerate modafinil's clearance from the body. This leads to lower plasma concentrations of modafinil and a shorter duration of action, potentially rendering a standard 200mg dose ineffective.
Examples of strong CYP3A4 inducers include certain antiepileptic drugs (e.g., carbamazepine, phenobarbital, phenytoin), rifampin (an antibiotic), and the herbal supplement St. John's wort.47 Apalutamide, used in cancer treatment, is an inducer of both CYP3A4 and CYP2C19 and has been noted to decrease modafinil levels.48 If an individual is taking such an enzyme-inducing medication, their genetically normal metabolism of modafinil could be significantly sped up (phenoconversion), leading to sub-therapeutic drug levels and a lack of response.44
Conversely, drugs that inhibit CYP3A4 or CYP2C19 could increase modafinil levels. While this might lead to side effects, it is less likely to cause non-response unless the higher concentration pushes the individual into an unfavorable part of a complex dose-response curve or causes intolerable side effects that mask any benefit.
Effects of Co-administration with Alcohol
The interaction between modafinil and alcohol is complex and not fully elucidated for all scenarios. General medical advice suggests limiting alcohol consumption while taking modafinil.22 One study in alcohol-dependent patients found that modafinil (300mg/day) improved self-reported state impulsivity but did not broadly reduce relapse. However, subgroup analysis revealed that modafinil prolonged time to relapse in patients with poor baseline response inhibition but, concerningly, increased the percentage of heavy drinking days and reduced abstinent days in patients with better baseline response inhibition.49 This suggests that for some individuals, combining modafinil with alcohol ... could lead to negative outcomes or alter the perceived efficacy of modafinil in unpredictable ways. An animal study investigating combination therapy post-stroke found that modafinil plus ethanol increased anxiety, although it did improve locomotor activity.50 While not directly applicable to a healthy individual feeling no effect from modafinil alone, it underscores that interactions occur.
Synergistic or Antagonistic Effects with Caffeine
Caffeine is a commonly consumed stimulant, and its interaction with modafinil is of particular interest. One study involving healthy adults subjected to 36 hours of sleep deprivation administered either 200mg of modafinil, 200mg of caffeine, or a placebo. A striking finding was that, compared with placebo, both modafinil and caffeine had insignificant effects on subjective measures of wakefulness or sleepiness at that time point, although both substances did modulate thalamic resting-state functional connectivity in the brain, albeit differently.51 This finding is highly relevant: if an individual is a regular and perhaps heavy consumer of caffeine, the additional subjective benefit from ... modafinil might be minimal, difficult to distinguish ... or even absent. Modafinil's subjective effects have been described as being closer to caffeine than to amphetamines.13 Thus, if the body is already adapted to a certain level of stimulation from caffeine, modafinil might not provide a sufficiently distinct or stronger sensation, leading to a perception of "no effect." This represents a pharmacodynamic interaction at the level of perceived effect, even if their precise neural mechanisms are not identical. An animal study, however, did show that both caffeine and modafinil could ameliorate anxious behavior and neuroinflammation during sleep deprivation.52
Crucial Check: Always consider an individual's entire regimen of medications, supplements, and lifestyle substance use (like alcohol and caffeine) when evaluating modafinil non-response, as these interactions can significantly alter efficacy.
The following table outlines significant drug interactions that could affect modafinil efficacy:
| Interacting Drug/Substance | Mechanism of Interaction | Effect on Modafinil Levels/Efficacy | Clinical Implication for Modafinil User | Key Supporting Sources |
|---|---|---|---|---|
| Strong CYP3A4 Inducers (e.g., carbamazepine, rifampin, St. John's Wort) | Induction of CYP3A4, a key enzyme in modafinil metabolism. | Decreased plasma concentrations of modafinil, potentially leading to sub-therapeutic levels and reduced or absent efficacy. | Perceived non-response to modafinil; therapeutic failure. | 47 |
| Strong CYP2C19 Inducers (e.g., apalutamide, rifampin) | Induction of CYP2C19, which may contribute to modafinil metabolism. | Decreased plasma concentrations of modafinil, potentially leading to sub-therapeutic levels and reduced or absent efficacy. | Perceived non-response to modafinil; therapeutic failure. | 48 |
| Alcohol | Complex CNS interactions; specific mechanisms affecting modafinil efficacy are not fully clear. | Variable effects; may alter perceived efficacy or lead to adverse outcomes, potentially masking modafinil's benefits. | Unpredictable response; potential for negative interactions; may contribute to perception of inefficacy. | 22 |
| Caffeine | CNS stimulant with some overlapping subjective effects with modafinil. | May mask or minimize the perceived subjective benefits of modafinil, especially in regular caffeine users. One study showed no significant subjective wakefulness benefit over placebo for either drug after prolonged sleep deprivation. | Perceived lack of additional benefit from modafinil; subjective non-response. | 13 |
These interactions highlight the importance of considering an individual's entire regimen of medications, supplements, and lifestyle substance use when evaluating a lack of response to modafinil.
7. Impact of Underlying Health Conditions
The presence of certain underlying medical or psychological conditions can significantly influence modafinil's effectiveness, sometimes leading to non-response or even paradoxical effects. The specific neurobiology of a disorder may interact with modafinil's mechanism of action in ways that are not seen in healthy individuals or in its approved indications.
Stroke:
In the acute phase of stroke, particularly in an ICU setting, modafinil monotherapy (at doses of 100-200mg) was found to be ineffective in improving wakefulness or Glasgow Coma Scale scores in one study. This lack of response was hypothesized to be due to post-stroke dopaminergic system dysfunction, which modafinil's primarily non-dopaminergic wakefulness mechanisms (in this context) might not adequately address.1 However, in stroke survivors experiencing persistent fatigue three or more months after their event, a 6-week course of 200mg modafinil daily significantly reduced fatigue and improved quality of life compared to placebo.53 This suggests a time-dependent and context-dependent efficacy in stroke.
Multiple Sclerosis (MS):
A meta-analysis of studies on modafinil for MS-related fatigue and excessive daytime sleepiness (EDS) found no significant beneficial effect of the drug on these symptoms as measured by scales like the Fatigue Severity Scale (FSS) or Modified Fatigue Impact Scale (MFIS).4 While some earlier, smaller, or open-label studies had suggested potential benefits for fatigue in MS2, the broader evidence from systematic reviews is less supportive.
Parkinson's Disease (PD):
Similar to MS, a meta-analysis indicated that modafinil did not have a significant effect on fatigue in patients with PD.4 Other studies have reported inconsistent findings regarding its utility for fatigue in this population.2
Traumatic Brain Injury (TBI):
For TBI-related fatigue, a meta-analysis did show a therapeutic effect of modafinil. However, for EDS in TBI, the results were heterogeneous and the meta-analysis did not demonstrate a significant overall benefit.4
Bipolar Disorder:
The use of modafinil in bipolar disorder presents a complex picture. In affectively stable patients with bipolar disorder, adjunctive modafinil (mean dose around 200mg) showed some preliminary cognitive benefits (in processing speed and verbal learning) and reduced daytime sleepiness. However, it was also associated with side effects including, paradoxically, fatigue and decreased energy in some participants, as well as potentially worsened sleep quality.18 Other research suggests modafinil may have efficacy as an adjunctive treatment for bipolar depression.2 The unique neurochemical environment in bipolar disorder may lead to these mixed or unexpected responses.
Attention-Deficit/Hyperactivity Disorder (ADHD):
Modafinil has not consistently demonstrated efficacy in treating adult ADHD, especially when compared to established treatments.8 While there is some evidence of efficacy in pediatric ADHD populations12, its role in adults remains uncertain.
Depression (Unipolar):
Some open-label studies and chart reviews have suggested that modafinil (e.g., mean dose 184mg) can be beneficial in alleviating fatigue and improving depressive symptoms, both as monotherapy and as an adjunctive treatment, with effects seen as early as 2 weeks and persisting at 3 months.2
Cancer-Related Fatigue:
Evidence for modafinil's efficacy in managing cancer-related fatigue is mixed, with some studies showing benefit and others not.2
Primary Brain Tumor Patients:
In a study of patients with primary brain tumors, modafinil (up to 400mg/day) did not demonstrate effects superior to placebo in managing symptoms like fatigue, motivation, or cognitive function.16
Baseline Matters: Modafinil's perceived effectiveness can be influenced by an individual's initial alertness. If already highly alert, the drug's impact might be negligible or absent, especially for cognitive enhancement goals.
Furthermore, an individual's baseline alertness and cognitive state can influence modafinil's perceived effects. Some researchers have suggested that positive outcomes in cognitive enhancement studies might be partly due to the inclusion of individuals with relatively low baseline alertness. In such cases, modafinil's primary wakefulness-enhancing effect could improve performance on tasks that are sensitive to decrements in alertness, rather than directly enhancing specific cognitive domains in already alert individuals.3 The general principle that catecholaminergic drugs may yield greater improvements in individuals with lower baseline performance has also been noted.46 If an individual is already functioning at a high level of alertness or cognitive capacity, the additional impact of modafinil may be negligible or absent.
These examples illustrate that the underlying pathophysiology of a given condition can significantly alter or even negate modafinil's typical actions. The drug's mechanisms, while effective for certain indications, may not be suitable or sufficient to address the specific neurobiological disturbances present in other disorders, leading to non-response or unexpected outcomes.
8. Dosage, Administration, and Product Integrity
Even if an individual has no specific genetic, pharmacokinetic, or pharmacodynamic reason for non-response, factors related to the dose administered, the way it's taken, the duration of trial, and the quality of the product itself can lead to a perceived lack of effect.
The Importance of Appropriate Dosage and Dosing Regimen
The typical therapeutic dose of modafinil for its approved indications, such as narcolepsy or OSAHS, is 200mg taken once daily in the morning. Physicians may adjust this dose based on individual response and tolerability, with some studies using doses up to 400mg or even 600mg per day.7 For conditions like fatigue, lower doses (e.g., 50-200mg) are sometimes explored.16
The dosing regimen can also matter. For instance, in patients with narcolepsy, a 400mg daily dose given in a split regimen (e.g., 200mg twice a day) was found to be potentially superior to a single 200mg or 400mg once-daily dose for maintaining wakefulness throughout the entire day, particularly in the evening.56
Important Advice: If modafinil seems ineffective, do not independently increase the dose. Always consult a healthcare professional for guidance on dosage adjustments or to explore other potential reasons for non-response.
If an individual feels that modafinil is not working after an adequate trial period, it is crucial not to independently increase the dose. Instead, consultation with a healthcare professional is recommended.22 In some clinical situations, physicians have adjusted doses (e.g., to 300mg) or the timing of administration for patients who were initially non-responsive to a standard dose, with some subsequently experiencing benefit.2 However, a 200mg dose is a standard and often effective starting point, and its lack of effect warrants careful consideration of other factors before assuming a simple need for dose escalation.
Realistic Expectations for Time to Effect and Trial Duration
Modafinil is absorbed relatively quickly, with peak plasma concentrations occurring 2 to 4 hours after oral administration.7 While some effects, like increased alertness, might be noticeable within this timeframe for responders, the full therapeutic benefit, especially for more complex symptoms like fatigue in chronic conditions or depressive symptoms, may take longer to become apparent. For example, one chart review noted improvements in depression rating scales after 2 weeks and 3 months of modafinil treatment.55 Clinical trials evaluating modafinil for conditions like post-stroke fatigue or symptoms in primary brain tumor patients have often used treatment periods of 6 weeks.16 Medical guidance often suggests that if modafinil is perceived as not working properly after "a few weeks" of consistent use, a physician should be consulted.22 An inadequate trial duration, where an individual expects immediate and dramatic effects and discontinues use prematurely, could lead to a false conclusion of non-response.
Concerns Regarding Counterfeit or Substandard Modafinil from Unverified Sources
Critical Warning: A significant reason for perceived modafinil ineffectiveness is the use of counterfeit or substandard products from unverified sources. These may contain little to no active ingredient.
A critical and often overlooked reason for modafinil appearing ineffective is the quality and authenticity of the product itself, particularly if it is obtained from sources other than a licensed pharmacy with a legitimate prescription. There is a significant issue with counterfeit and substandard modafinil being sold online.57 Laboratory analyses of modafinil products purchased from unverified online sources have revealed that they can contain significantly less of the active pharmaceutical ingredient than stated on the label. In some cases, the actual modafinil content has been found to be as low as 45.5% to 80.5% of the labeled dose.23
If an individual is unknowingly taking a product that is supposed to contain 200mg of modafinil but in reality contains only 90mg or less, it is highly plausible that this sub-potent dose would not produce any noticeable effect, especially if that individual also has other factors contributing to a reduced response (e.g., specific genetic makeup, rapid metabolism). This issue of product integrity is a crucial practical consideration. The variability in dosage and purity from non-pharmaceutical or illicitly sourced modafinil not only leads to inefficacy but also poses potential health risks due to unknown contaminants or inconsistent dosing.23
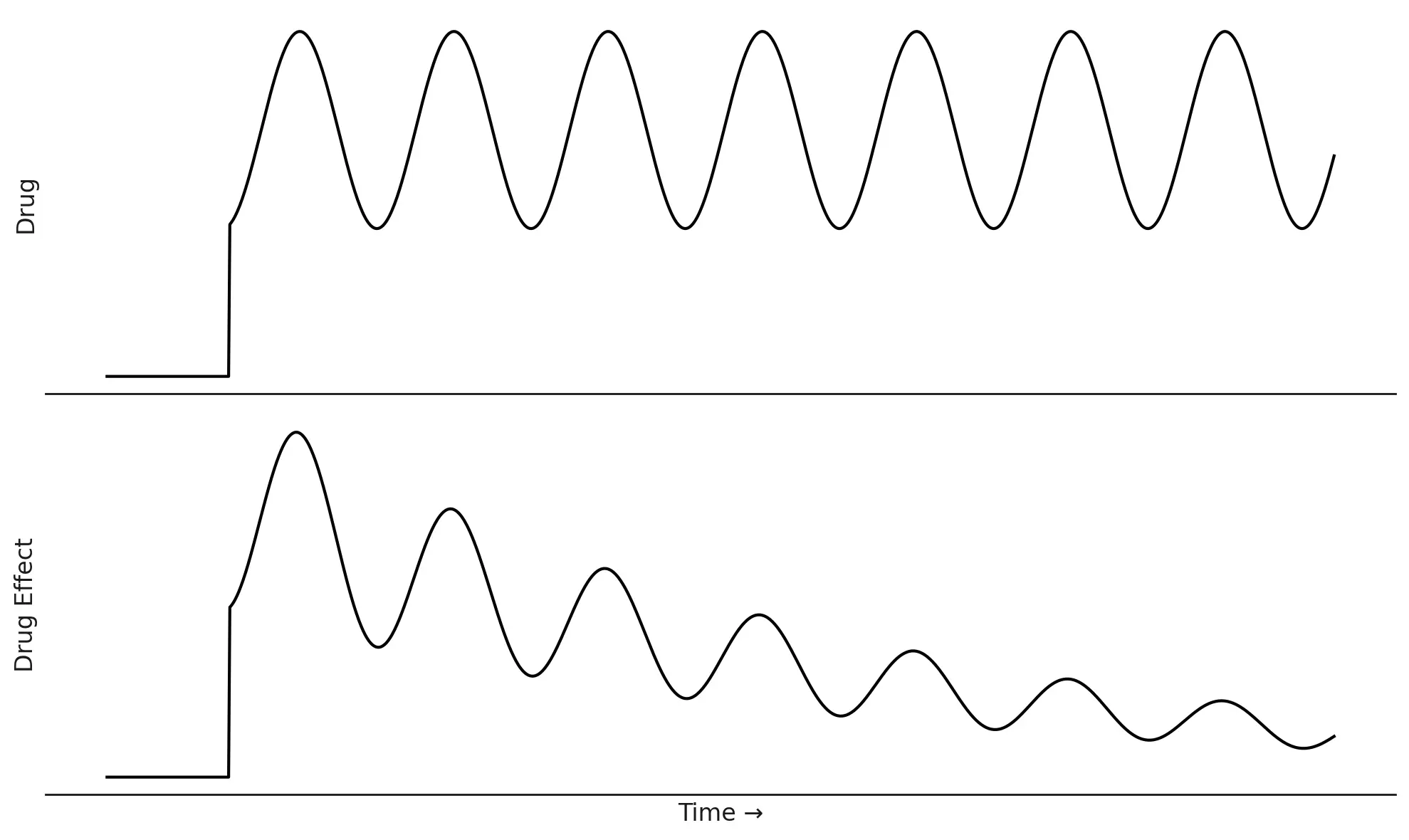
9. The Phenomenon of Tolerance
Tolerance occurs when the response to a drug diminishes over time with repeated administration, such that higher doses are needed to achieve the same effect initially produced by a lower dose.8
Potential for Tolerance Development
While extensive clinical research involving therapeutic use of modafinil for conditions like narcolepsy (with treatment durations up to 40 weeks) has generally not identified tolerance as a common adverse effect8, the potential for tolerance development exists, particularly under certain circumstances. Tolerance appears to be more of a concern with non-medical (off-label) use, employment of higher-than-prescribed doses, or very prolonged administration.8
There are case reports in the literature describing individuals who, often in the context of self-medication or seeking cognitive enhancement, escalated their modafinil dosage significantly over time—for example, from an initial 100-200mg to 800-1000mg per day. These escalations were often driven by a diminishing effect of the drug (tolerance) and sometimes accompanied by cravings or withdrawal-like symptoms upon attempting to reduce or stop the drug.59 One source cites a European Medicines Agency (EMA) study which purportedly found that 70% of modafinil users required an increase in their initial prescription dose over a 12-month period to maintain its effects, although the primary source for this specific EMA study finding requires careful verification.58
Mechanisms of Tolerance
The precise neurobiological mechanisms underlying modafinil tolerance are not fully understood but are thought to involve neuroadaptations in the dopamine and norepinephrine systems, among others, in response to chronic drug exposure.8 These adaptations could include changes in receptor density (downregulation), receptor sensitivity (desensitization), alterations in neurotransmitter synthesis or release, or changes in intracellular signaling pathways.
Tolerance vs. Non-Response: It's important to distinguish if an individual never felt effects (primary non-response) or if effects faded over time (tolerance). Unnoticed early tolerance can mimic primary non-response.
Distinguishing Tolerance from Primary Non-Response
It is important to distinguish between tolerance and primary non-response. Primary non-response occurs when an individual never experiences the expected or desired effects from the drug, even from the initial doses. Tolerance, on the other hand, implies that there was an initial response that subsequently faded with continued use. The query regarding an acquaintance who "doesn't feel anything" from 200mg modafinil daily suggests primary non-response.
However, the line can sometimes blur. If an individual experimented with modafinil intermittently or at varying doses before settling into a consistent daily regimen, early and perhaps unnoticed tolerance development could have occurred. In such a scenario, when they begin a "formal" trial of 200mg daily, their baseline might already be a slightly tolerant state, leading to the perception that the 200mg dose is ineffective from the outset. This is more speculative but remains a possibility in the context of off-label or experimental use patterns.
10. Subjective Experience: When "Not Working" is a Matter of Perception
The subjective experience of taking a drug is a complex interplay of its pharmacological effects, an individual's physiological state, their expectations, and their interpretation of any perceived changes. For modafinil, several aspects of its subjective effects profile could lead an individual to conclude it is "not working," even if it is producing some neurobiological changes.

Effects on Response Latency vs. Accuracy in Cognitive Tasks
Some research in healthy volunteers has shown that modafinil can increase response latency, meaning it can slow down reaction times in certain types of cognitive tasks. This slowing may occur even if cognitive accuracy is maintained or, in some paradigms, slightly improved.3 For an individual seeking cognitive enhancement with the expectation of becoming mentally "sharper" or "quicker," a perceptible slowing of thought processes or response speed could be interpreted negatively, as the drug failing to meet their desired outcome, or even as a cognitive impairment.3
Absence of Euphoric Effects Unlike Classical Stimulants
A defining characteristic of modafinil, distinguishing it from classical psychostimulants like amphetamines or methylphenidate, is its general lack of euphoric effects or a "high" at therapeutic doses.7 The subjective psychoactive effects of modafinil are often described as being more subtle and qualitatively different, sometimes likened to those of caffeine.13 If a user, particularly one using modafinil off-label for cognitive enhancement or increased productivity, anticipates a pronounced mood lift, a surge of energy, or an intense feeling of stimulation similar to that produced by other stimulants, they are likely to be disappointed by modafinil's more nuanced profile. This mismatch between expectation and the actual subjective experience can easily lead to the conclusion that the drug "doesn't feel like anything" or is "not working." The absence of a dramatic "kick" might be interpreted as an absence of any effect at all.
Individual Variability in Subjective Effects
The subjective effects of modafinil are not uniform across all users. While some studies report that individuals feel more alert, attentive, energetic, or experience increased task enjoyment while on modafinil24, others find minimal impact on overall mood.25 In specific populations, such as individuals with bipolar disorder, paradoxical effects like increased fatigue have even been reported as side effects.18
Furthermore, as previously mentioned, a study involving 36 hours of sleep deprivation found that both modafinil (200mg) and caffeine (200mg) had insignificant effects on subjective ratings of wakefulness or sleepiness when compared to placebo, despite inducing measurable changes in brain functional connectivity.51 This discrepancy between objective neurobiological activity and subjective perception is crucial. An individual might not "feel" significantly more awake or focused, even if the drug is exerting some physiological action.
Perception is Key: If modafinil doesn't produce the *type* or *intensity* of sensation anticipated, it may be deemed ineffective, regardless of subtle physiological or cognitive effects it might be producing.
Collectively, these subjective factors mean that an individual's assessment of whether modafinil is "working" is heavily influenced by what they expect to feel and how they interpret the subtle (or absent) changes in their mental and physical state. If the drug does not produce the specific type of sensation or level of intensity anticipated, it may be deemed ineffective, irrespective of any underlying physiological or subtle cognitive effects it might be producing.
11. Conclusion: Navigating Modafinil Non-Response
The phenomenon of modafinil failing to produce a noticeable effect in certain individuals, even at a standard therapeutic dose of 200mg per day, is not attributable to a single, simple cause. Instead, it arises from a complex and often intertwined array of factors. These include:
- Pharmacokinetic variability: Differences in how an individual's body absorbs, distributes, metabolizes (particularly via CYP450 enzymes like CYP3A4 and CYP2C19), and excretes the drug can lead to sub-therapeutic plasma concentrations.
- Genetic predispositions: Polymorphisms in genes such as COMT (critically affecting cognitive response versus wakefulness), as well as variations in genes encoding CYP enzymes (e.g., CYP2C19, CYP3A4) and dopamine system components (e.g., DAT1, DRD2), can significantly alter drug metabolism and target sensitivity.
- Pharmacodynamic differences: Baseline neurotransmitter levels (e.g., the "dopamine tone" and the inverted U-shaped response curve), receptor sensitivity, and the unique "atypical" stimulant profile of modafinil contribute to varied individual responses.
- Drug interactions: Concomitant use of other medications (especially CYP enzyme inducers), alcohol, or even high doses of caffeine can interfere with modafinil's efficacy or alter its perceived effects.
- Underlying health conditions: The specific neurobiology of certain neurological or psychiatric disorders (e.g., acute stroke, MS, aspects of bipolar disorder) can render modafinil ineffective or lead to paradoxical outcomes.
- Product integrity and administration: Substandard or counterfeit products with insufficient active ingredient, or an inadequate trial duration or suboptimal dosing strategy, can result in a lack of perceived effect.
- Tolerance: While less common in strictly therapeutic use for approved indications, tolerance can develop, especially with non-medical use or dose escalation, diminishing the drug's effects over time.
- Subjective expectations and perception: A mismatch between an individual's expectations (often based on experiences with classical stimulants) and modafinil's more subtle subjective effects profile, or negative perceptions of effects like increased response latency, can lead to the conclusion that the drug is not working.
A 200mg daily dose of modafinil is a standard therapeutic starting point for its approved indications, but its universal effectiveness cannot be assumed. The path to understanding why modafinil may not be working for a specific individual requires a systematic and personalized consideration of all these potential contributing factors.
Professional Consultation is Key: If modafinil is perceived to be ineffective, it is strongly recommended to consult a qualified healthcare professional. Self-adjusting dosages or sourcing from unverified channels can be risky.
It is strongly recommended that if an individual, such as the acquaintance mentioned in the user's query, perceives modafinil to be ineffective, they should consult a qualified healthcare professional. Self-adjusting the dosage, attempting to source the drug from unverified channels, or combining it with other substances to achieve an effect can be risky and is not advised. A healthcare professional can help to explore the potential reasons for non-response by reviewing concomitant medications, considering underlying medical or psychological conditions, discussing realistic expectations of therapy, and potentially exploring other diagnostic or therapeutic avenues. The information provided in this report is for educational purposes and does not constitute medical advice.
Works Cited
- Amantadine and Modafinil as Neurostimulants Following Acute ..., accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7239352/
- MODAFINIL TREATMENT FOR FATIGUE IN PATIENTS WITH HIV/AIDS: A PLACEBO CONTROLLED STUDY - PMC, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2892004/
- Modafinil Increases the Latency of Response in the Hayling ..., accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4229110/
- Efficacy of Modafinil on Fatigue and Excessive Daytime Sleepiness ..., accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3849275/
- Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/26381811/
- Modafinil, an atypical CNS stimulant? - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/38467484/
- Modafinil : A Review of its Pharmacology and Clinical Efficacy in the Management of Narcolepsy - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/27521015/
- Modafinil - Wikipedia, accessed May 31, 2025, https://en.wikipedia.org/wiki/Modafinil
- Modafinil as a Catecholaminergic Agent: Empirical Evidence and Unanswered Questions - PMC - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3791559/
- Modafinil, an atypical CNS stimulant? - PMC, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC12004278/
- Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/24109471/
- Modafinil - StatPearls - NCBI Bookshelf, accessed May 31, 2025, https://www.ncbi.nlm.nih.gov/books/NBK531476/
- Subjective effects of modafinil, a new central adrenergic stimulant in healthy volunteers: a comparison with amphetamine, caffei, accessed May 31, 2025, https://core-cms.cambridgecore.org/.../subjective_effects_of_modafinil...pdf
- Practical Use and Risk of Modafinil, a Novel Waking Drug - PMC - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3286657/
- Mechanisms of modafinil: A review of current research - PMC, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2654794/
- The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3779045/
- Modafinil Effects: Genetic reason for no response - Genetic Lifehacks, accessed May 31, 2025, https://www.geneticlifehacks.com/modafinil-response/
- Modafinil's effects on cognition and sleep quality in affectively-stable ..., accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC10507316/
- Modafinil: Uses, Interactions, Mechanism of Action | DrugBank Online, accessed May 31, 2025, https://go.drugbank.com/drugs/DB00745
- Effects of Modafinil and Sleep Loss on Physiological Parameters - Oxford Academic, accessed May 31, 2025, https://academic.oup.com/milmed/article/173/11/1092/4265797
- Long-term efficacy and safety of modafinil (PROVIGIL((R))) for the treatment of excessive daytime sleepiness associated with narcolepsy - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/10828434/
- Modafinil (oral route) - Mayo Clinic, accessed May 31, 2025, https://www.mayoclinic.org/drugs-supplements/modafinil-oral-route/description/drg-20064870
- The Issue of “Smart Drugs” on the Example of Modafinil: Toxicological Analysis of Evidences and Biological Samples - MDPI, accessed May 31, 2025, https://www.mdpi.com/2039-4713/15/1/15
- Cognitive enhancing effects of modafinil in healthy volunteers - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/12417966/
- Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers - PMC, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC3485563/
- The Use and Impact of Cognitive Enhancers among University Students: A Systematic Review - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC8000838/
- (PDF) Pharmacogenetics of Modafinil After Sleep Loss: Catechol-O ..., accessed May 31, 2025, https://www.researchgate.net/publication/23501009...Sleep
- Clinical pharmacokinetic profile of modafinil - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/12537513/
- PROVIGIL® (modafinil) Tablets [C-IV] - accessdata.fda.gov, accessed May 31, 2025, https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20717se1-008_provigil_lbl.pdf
- PROVIGIL® (modafinil) Tablets [C-IV] Rx Only DESCRIPTION - accessdata.fda.gov, accessed May 31, 2025, https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020717s030s034s036lbl.pdf
- Drug Interaction Report: Synthroid, modafinil - Drugs.com, accessed May 31, 2025, https://www.drugs.com/interactions-check.php?drug_list=1463-869,1647-0&professional=1
- Evaluation of modafinil as a perpetrator of metabolic drug-drug interactions using a model informed cocktail reaction phenotyping trial protocol - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/29178272/
- Modafinil - LiverTox - NCBI Bookshelf, accessed May 31, 2025, https://www.ncbi.nlm.nih.gov/books/NBK548274/
- Genes, Cognition and Brain through a COMT Lens - PMC - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC2760675/
- 1312 - Gene ResultCOMT catechol-O-methyltransferase [ (human)] - NCBI, accessed May 31, 2025, https://www.ncbi.nlm.nih.gov/gene/1312
- Which Dopamine Polymorphisms Are Functional? Systematic ..., accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/31303260/
- Genetic polymorphisms of DAT1 and COMT differentially associate ..., accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/24625311/
- Cleveland Clinic Laboratories - Duke University, accessed May 31, 2025, https://dcricollab.dcri.duke.edu/.../2.1-Cleveland-Clinic-CYP2C19-test.pdf
- CYP2C19 - Wikipedia, accessed May 31, 2025, https://en.wikipedia.org/wiki/CYP2C19
- A view of response and resistance to atomoxetine treatment in children with ADHD: effects of CYP2C19 polymorphisms and BDNF levels | Request PDF - ResearchGate, accessed May 31, 2025, https://www.researchgate.net/publication/360268479...
- Diazepam Therapy and CYP2C19 Genotype - Medical Genetics Summaries - NCBI, accessed May 31, 2025, https://www.ncbi.nlm.nih.gov/books/NBK379740/
- Developmental Changes in the Expression and Function of Cytochrome P450 3A Isoforms: Evidence from In Vitro and In Vivo Investigations - ResearchGate, accessed May 31, 2025, https://www.researchgate.net/publication/235797396...
- Quantitative Prediction of CYP3A4 and CYP3A5‐Mediated Drug Interactions | Request PDF, accessed May 31, 2025, https://www.researchgate.net/publication/334755764...
- Addressing phenoconversion: the Achilles' heel of personalized medicine - PMC, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC4309629/
- Participation of Dopamine D1 and D2 Receptors in the Rapid-Onset Behavioral Sensitization to Modafinil, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC6421293/
- Methamphetamine-induced adaptation of learning rate dynamics depend on baseline performance - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11257491/
- Provigil Interactions: Other Medications, Alcohol, and More - Healthline, accessed May 31, 2025, https://www.healthline.com/health/drugs/provigil-interactions
- Provigil (modafinil) dosing, indications, interactions, adverse effects, and more - Medscape, accessed May 31, 2025, https://reference.medscape.com/drug/provigil-modafinil-343000
- Effect of modafinil on impulsivity and relapse in alcohol dependent patients: a randomized, placebo-controlled trial - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/23141152/
- Behavioral Changes in Combination Therapy of Ethanol and Modafinil on Rats Focal Cerebral Ischemia - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC7502185/
- Caffeine and modafinil modulate the effects of sleep deprivation on thalamic resting-state functional connectivity: A double-blind pilot study - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/39137663/
- Caffeine and Modafinil Ameliorate the Neuroinflammation and Anxious Behavior in Rats during Sleep Deprivation by Inhibiting the Microglia Activation - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC5863523/
- MIDAS (Modafinil in Debilitating Fatigue After Stroke) - American Heart Association Journals, accessed May 31, 2025, https://www.ahajournals.org/doi/10.1161/strokeaha.116.016293
- Use of adjunctive stimulants in adult bipolar depression - Oxford Academic, accessed May 31, 2025, https://academic.oup.com/ijnp/article/16/1/55/629710
- A retrospective chart review of the effects of modafinil on depression as monotherapy and as adjunctive therapy - ResearchGate, accessed May 31, 2025, https://www.researchgate.net/publication/7710289...
- Dosing regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsy - PubMed, accessed May 31, 2025, https://pubmed.ncbi.nlm.nih.gov/14520165/
- Generic MODAFINIL INN equivalents, drug patent expiry and freedom to operate, accessed May 31, 2025, https://www.drugpatentwatch.com/p/generic-api/MODAFINIL
- Modafinil Tolerance: How to Help Yourself | Windward Way Recovery, accessed May 31, 2025, https://windwardway.com/withdrawal/detox/modafinil/tolerance/
- Development of Modafinil Dependence Following Self-medication for Sexual Dysfunction: A Case Series - PMC - PubMed Central, accessed May 31, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11938520/